
entimICE® FastTrack
The first cloud-native compliant SCE for clinical research

entimICE FastTrack statistical computing environment significantly increases the efficiency of clinical data science processes, managing constantly growing study pipelines, resource bottlenecks, and ever-increasing regulatory requirements.
entimICE FastTrack is ready today for data from decentralized clinical trials and various sources, and fully supports real-time analysis and reporting along with the use of AI/ML.
distinct user requirements fulfilled
months average time to go-live
entimICE users worlwide
Your benefits
A meaningful difference in your clinical operations

Accelerated time to market
entimICE FastTrack significantly increases the efficiency of clinical data science processes, managing growing study pipelines and avoiding resource bottlenecks.

Risk reduction and compliance
Built by domain experts for today’s clinical challenges, the enterprise-grade platform ensures reliable processing, traceable outcomes, and effortless compliance.

Scalability for growth
Designed for sponsors, CROs, and biotechs of all sizes, the platform adapts to your needs and scales with your portfolio, managing constantly growing study pipelines and evolving operational demands.

Future-proof operations
entimICE FastTrack is ready today for data from decentralized clinical trials and various sources, fully supporting real-time analysis, reporting, and the use of AI/ML—ensuring your organization stays ahead of evolving trial models and technology trends.

Operational efficiency
With an intuitive, language-agnostic environment, entimICE FastTrack streamlines code development, automates data transformation, and accelerates deliverable creation through integrated, end-to-end workflows.

Cost savings
Centralized governance and actionable workflows reduce reliance on manual, fragmented processes thus improving visibility, freeing expert resources, and lowering operational and validation costs.
entimICE FasTrack in detail
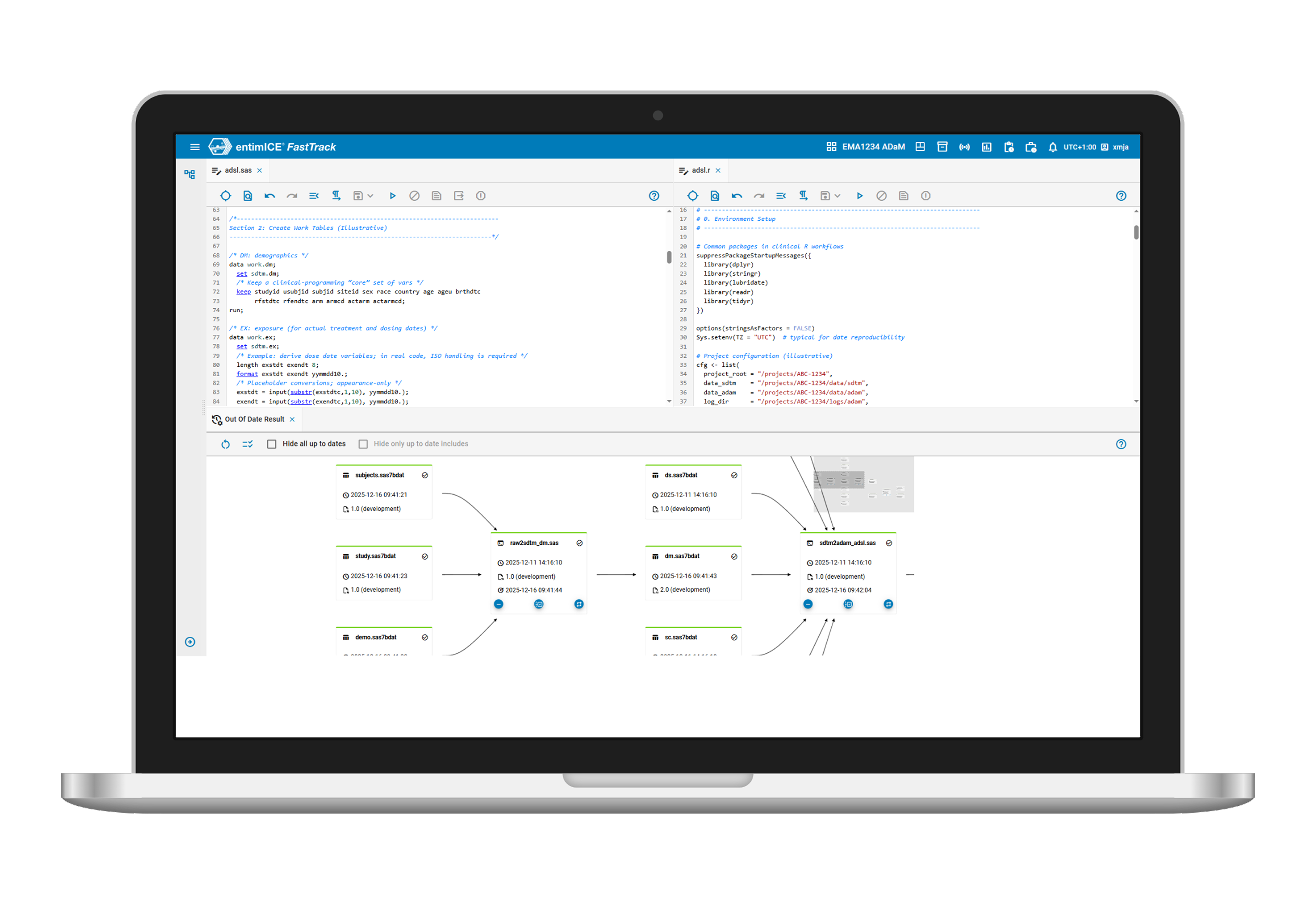
- Language-agnostic and flexible UI
Work natively in SAS, Posit (RStudio), or Python through a configurable, non-blocking UI with flexible dashboards tailored to individual workflows. - Traceable
Automatic traceability of program executions and clinical data flow, enabling the viewing of the “bigger picture” through interactive graphs and automated re-runs for outdated outputs. - Quality control
Built-in robust workflows from development to production facilitate lifecycle management and validation, ensuring quality control, documentation, and signature-driven approval. - Automation
Automation tools, including program run sequences and a job manager, eliminate repetitive tasks. The open API enables integration and automation across external systems and enterprise workflows.
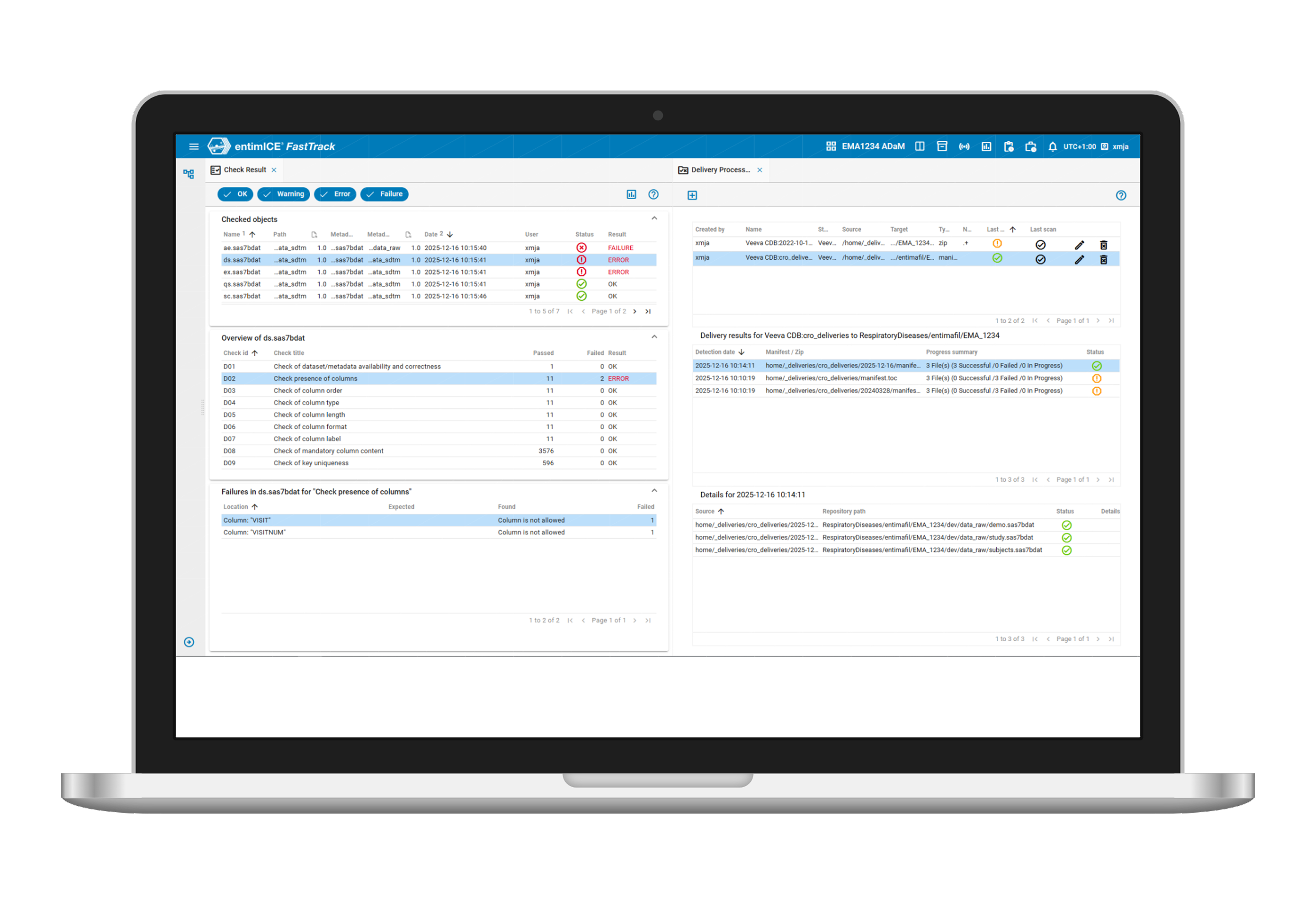
- Any data source
Access any clinical, legacy, or real-world data and quickly find relevant artifacts—including programs, datasets, and outputs—through intuitive search and navigation. - Connect upstream
Open REST APIs enable connectivity with upstream data sources for controlled access to data and metadata in entimICE FastTrack. - Ensure security
Secure, role-based access to data ensures data security and compliance. - Omnivorous
Ingest structured and unstructured data in real time with automated quality checks. - Granular standards
Manage data standards centrally and apply them at global or study levels.
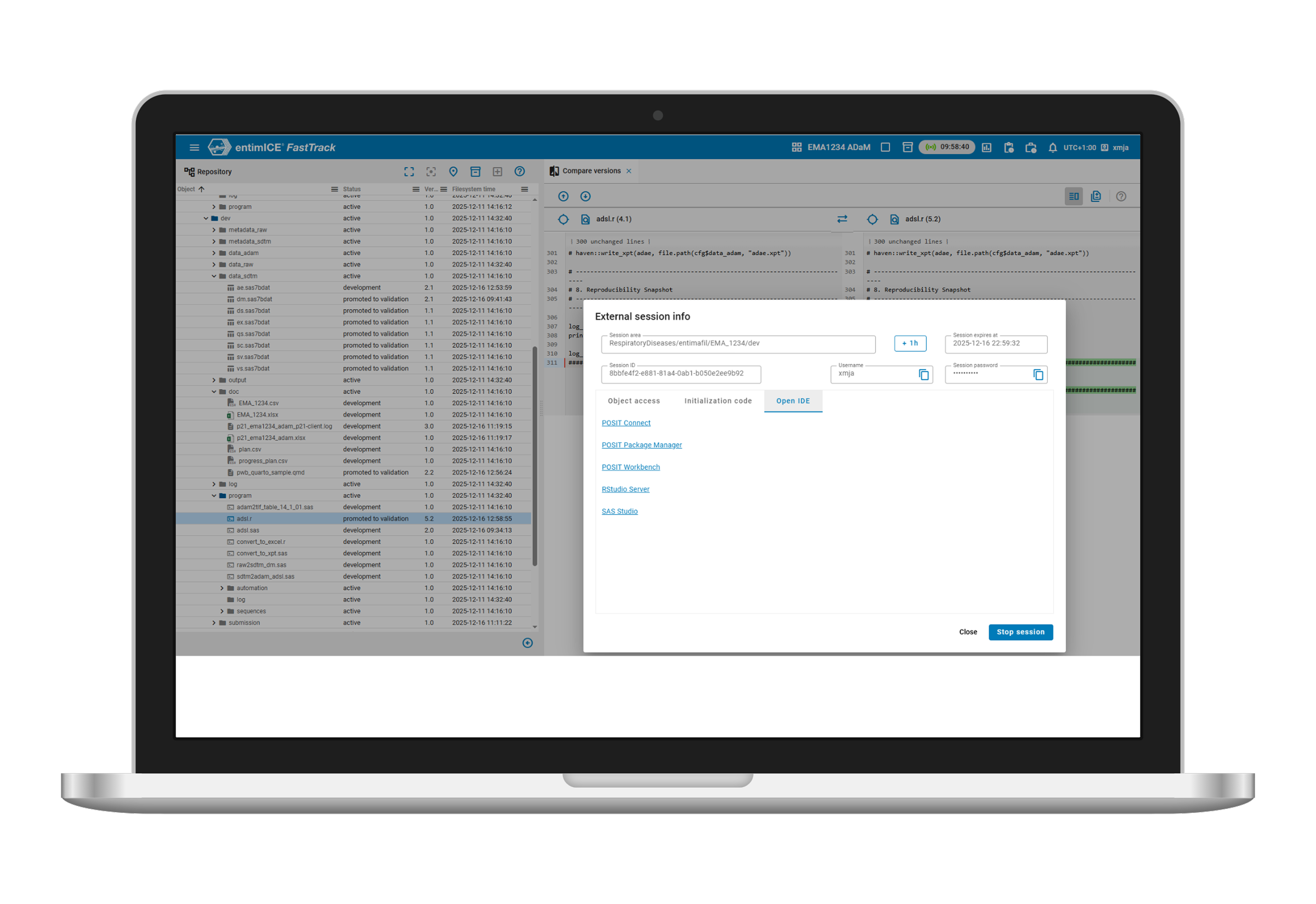
- Integrated clinical platform
entimICE FastTrack is a cloud-native, integrated clinical platform that combines the power of a Statistical Computing Environment, open-source tools and out-of-the box workflows to stream analysis and reporting activities. - Microservices architecture
Based on microservices with significantly shorter test and validation cycles, enabling scalability for business growth. - Easy integration
Standardized and validated REST APIs allow easy integration of any third-party commercial or open-source tool with entimICE FastTrack. - Metadata support
Enhanced study-specific metadata and terminology support improve data standardization, validation and review processes.
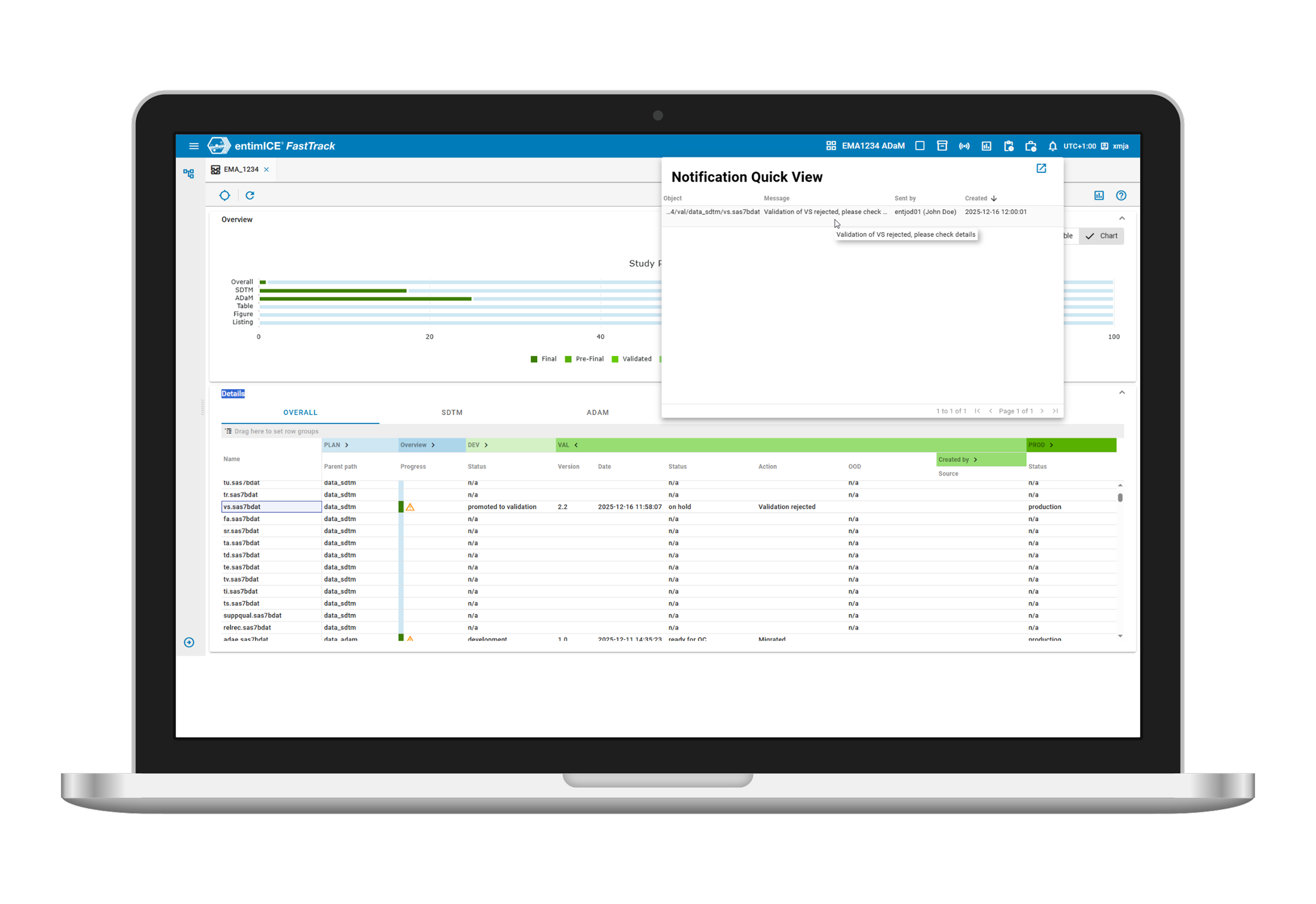
- Cloud Technology
entimICE FastTrack is a cloud-native, next-generation Statistical Computing Environment based on microservices, enabling scalability for business growth. - Easy overviews
Study Progress dashboards and reports provide a complete overview of activities in all ongoing studies and enables active task and deliverable management. - Managing processes
Built-in workflows ensure the process management of all objects covering lifecycle management and QC framework for submission review. - Modern tracking
Issue tracking at all levels with automatic notifications with full audit trail enable faster query resolution, eliminating the need for spreadsheet-based tracking. - Team collaboration
Collaborate with study teams to resolve programming and source data discrepancies, and with medical writers to streamline clinical data review.
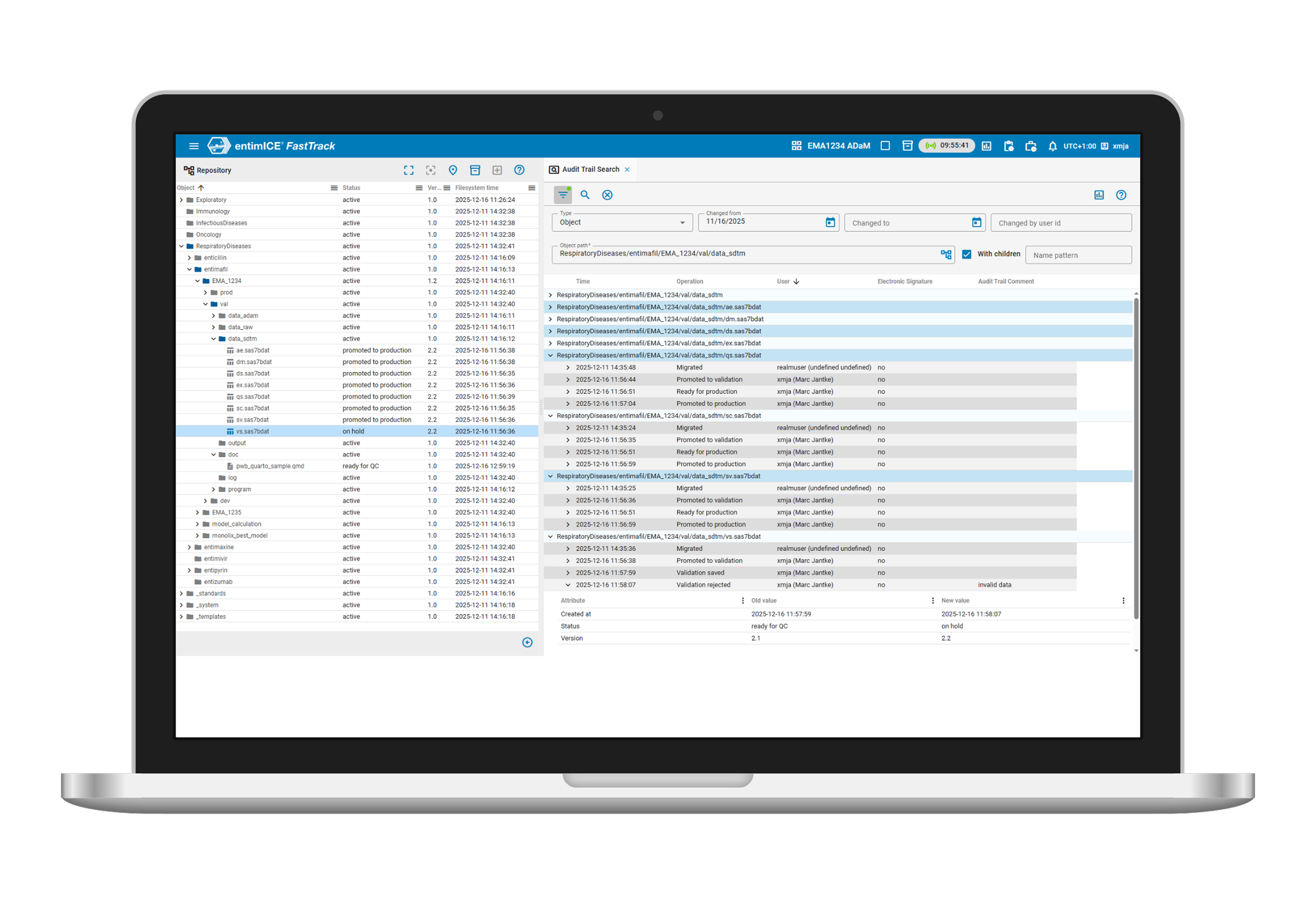
- Meet key standards
Automatic end-to-end traceability and effortless compliance with regulatory requirements, including FDA 21 CFR Part 11, EU Annex 11 and ICH GxP. - Find what you need
Flexible search and reporting engine so you can easily find relevant information from version control, audit trails, object history and execution traces. - Assign permissions
Flexible role based permission model that enables team to manage access control for internal users and partners with confidence. - Sign and audit workflows
Electronic signatures and tamper-proof audit trails log every action and decision, ensuring full accountability, transparency, and inspection readiness.
Use cases
FAQs
entimICE FastTrack is a cloud-ready statistical computing environment purpose-built for clinical development. Unlike traditional SCEs that focus solely on code execution and audit trails, entimICE FastTrack offers a modular platform for programming, data integration, version control, automation, and compliance—all in a single, traceable environment. It supports multi-language workflows (R, SAS, Python) and enables end-to-end visibility across study programming activities, making it fit for future and GxP-compliant by design.
entimICE FastTrack offers built-in traceability through audit trails, role-based access controls, automatic metadata capture, and seamless versioning of datasets, code, outputs, and specifications. It enables easy generation and validation of submission packages (e.g., SDTM, ADaM, define.xml, aCRF, SDRG) and integrates with tools like Pinnacle 21. Compliance with 21 CFR Part 11 and other regulatory guidelines is maintained through secure infrastructure, activity logs, and electronic signatures where required.
Yes. entimICE FastTrack is built with interoperability in mind. It supports API-based integrations with major EDC platforms, lab systems, real-world data sources, and metadata repositories. Our ingestion framework enables you to centralize diverse data types—EDC, non-EDC, lab, biomarkers, and RWD—into a unified, accessible layer for downstream analysis.
Yes. entimICE FastTrack enables robust study-level tracking by organizing deliverables, milestones, and timelines across clinical projects. Built-in interactive features allow teams to monitor execution status, review versioned outputs, and maintain visibility into submission readiness. Through collaborative workspaces, user activity logs, and status indicators, teams stay aligned and accountable throughout the study lifecycle.
entimICE FastTrack can be deployed in private cloud (AWS, Azure), or on-premises environments, depending on your IT policies. Security is a top priority—the platform includes encryption in transit, strict access controls, regular penetration testing, and compliance with global standards such as ISO/IEC 27001. Role-based permissions ensure data is accessed only by authorized users.
Have a project in mind?
Discover how our solution can streamline your workflows and elevate your results—contact us today to learn how it works for your organization.




